02188673652
info@tehransulphur.com
Buy Sodium Sulfide in Iran | Affordable Price & High Purity Guaranteed
Sodium Sulfide (Na₂S) is a highly reactive chemical compound available in various forms such as liquid, flakes, and lumps. Due to its unique properties, it plays a significant role in a wide range of industries.
When exposed to water, moisture, or air, sodium sulfide reacts rapidly and may release hydrogen sulfide gas (H₂S), which is toxic and hazardous. Therefore, it must be stored in a dry environment, away from moisture and direct sunlight.
This compound is widely used in various industries due to its reactivity and versatility. In the mining industry, it is used for sulfidizing and increasing the hydrophobicity of minerals during flotation processes. In the leather industry, it serves as a dehairing and sulfitizing agent, and in the textile industry, it functions as a bleaching agent.
Additionally, sodium sulfide plays a key role in kraft pulping in the paper industry, detergent production, paint manufacturing, water treatment, and oil extraction.
Its high reactivity and broad range of applications make sodium sulfide a vital component in many industrial processes. However, strict safety precautions must be followed when handling this substance to minimize risks and ensure optimal performance.
List
Chemical Formula and Structure of Sodium Sulfide
Molecular Formula: Na₂S
Crystal Structure: Sodium sulfide appears as a colorless to pale yellow solid and is available in various forms, including anhydrous, crystalline, and liquid types.
Composition: Sodium sulfide consists of two sodium ions (Na⁺) and one sulfide ion (S²⁻).

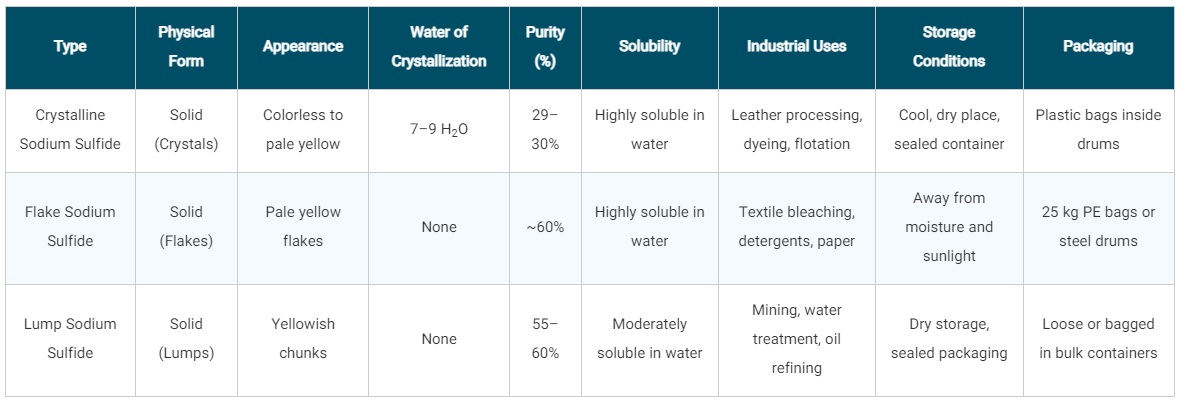
Types of Sodium Sulfide
1. Crystalline Sodium Sulfide
In its crystalline form, each molecule of sodium sulfide is associated with 7 to 9 molecules of water of crystallization. This type typically has a purity of around 29–30%.
2. Flake Sodium Sulfide
During the final stage of production, sodium sulfide is processed and cut into flake-like pieces. In this form, it generally has a purity of approximately 60%.
3. Lump (Broken) Sodium Sulfide
This type is obtained by breaking down larger dried chunks of sodium sulfide into smaller lumps. It usually has a purity ranging from 55% to 60%.

Sodium Sulfide Consumption and Export
The domestic consumption of sodium sulfide in Iran is considerably high. However, there are no restrictions on its export, and if produced with sufficient quality and offered at competitive prices, it holds strong potential in international markets.
Major global consumers of sodium sulfide include Germany, the United States, and France. Currently, due to geographic proximity and ease of transportation, Iran primarily exports sodium sulfide to neighboring countries such as Turkey, former Soviet states, and Iraq.

Sodium Sulfide – A Strong-Smelling Solid
Sodium sulfide (also known as disodium sulfide or sulfur sodium) is a white, water-soluble solid that can create alkaline solutions when dissolved. Both sodium sulfide and its hydrates can release hydrogen sulfide gas (H₂S) when exposed to air. This gas has a very strong and unpleasant odor, often compared to rotten eggs.
Industrial-grade sodium sulfide is typically yellow to reddish-brown in color due to the presence of polysulfides.
Production Methods of Sodium Sulfide
There are three main industrial methods for producing sodium sulfide:
Reduction of Sodium Sulfate with Coal
Reaction of Hydrogen Sulfide with Sodium Hydroxide (Caustic Soda)
Electrolysis of Sodium Chloride and Reaction of Sodium Amalgam with Sulfur
The choice of production method depends on factors such as availability of raw materials, process type, product quality, reaction efficiency, and economic feasibility.
1. Reduction of Sodium Sulfate with Coal
This method uses sodium sulfate and coal as the primary raw materials. These are mixed in a 2:1 ratio, and approximately 5% sodium carbonate (Na₂CO₃) is added to:
Accelerate the reaction process
Neutralize any free acid in the mixture
The blend is placed in a reverberatory furnace at 1000°C for about 2 to 2.5 hours to complete the reduction reaction.
The resulting product, known as black ash, is cooled, crushed, and washed multiple times with hot water. The sodium sulfide is then extracted at around 50°C.
This method yields yellow to light brown sodium sulfide. If white crystalline sodium sulfide is desired, 0.5% sodium cyanide (NaCN) can be added to the solution before crystallization.
2. Reaction of Hydrogen Sulfide with Sodium Hydroxide
In this process, 38% sodium hydroxide solution is injected into a tower where it reacts with hydrogen sulfide gas (H₂S). The initial product formed is sodium hydrosulfide (NaHS).
Reaction conditions:
Temperature: 50°C
Pressure: 3 atm
This intermediate (NaHS) can either be:
Collected, concentrated, and sold as a separate product
Or further reacted with additional sodium hydroxide to form sodium sulfide
Final Processing into Flake Sodium Sulfide
The resulting sodium sulfide solution is passed through evaporators to remove vapor. The concentrate is then pumped into a cooling drum where it forms a layer on a cold-water-cooled cylinder.
As the sodium sulfide cools and crystallizes on the cylinder surface, a blade scrapes it off into thin flakes. These flakes are then sent to the flake cutting machine, packaged into 25–50 kg bags, and stored in the warehouse.

Safety Information and Handling Precautions for Sodium Sulfide
Sodium sulfide is a highly alkaline and reactive compound that can cause severe irritation and damage upon contact with the skin or eyes.
In case of skin contact, especially with sensitive areas like the face or eyes, it is recommended to neutralize the affected area with a weak acid-based substance (such as a drink containing food-grade acid like soda) and seek immediate medical attention.
Do not rinse with plain water only, as this may not sufficiently neutralize the chemical.
Toxic Gas Emission
One of the major hazards of sodium sulfide is its ability to release toxic hydrogen sulfide gas (H₂S) when exposed to moisture or acids. This gas is highly dangerous and has a strong, unpleasant odor similar to rotten eggs. Exposure can lead to respiratory issues, nausea, or even asphyxiation in high concentrations.
Storage and Fire Safety Precautions
Always store sodium sulfide in airtight, moisture-resistant containers.
Keep the material in a dry, cool, and well-ventilated area, away from sources of heat, open flames, and oxidizing agents.
Use protective gear such as gloves, goggles, and masks when handling this chemical.
Applications of Sodium Sulfide
Some of the most important uses and applications of sodium sulfide include the following:
In the leather industry, sodium sulfide is used as a depilating agent to remove wool and hair from animal skins. It helps separate the hair from the hide.
In the paper industry, sodium sulfide plays a vital role in the kraft process, where it aids in producing wood pulp by separating cellulose fibers, leading to paper production.
A third use of sodium sulfide is its function as a very strong reducing agent. In many industrial reactions, it is used to reduce metal oxides.
In mining, sodium sulfide is used to assist in metal extraction. It adheres to mineral ores and helps separate other materials from the ore, playing an important role in the extraction of copper and lead.
Other uses of this chemical include applications in the textile industry, water treatment, hydrogen sulfide gas production, and pharmaceutical compounds, among others.
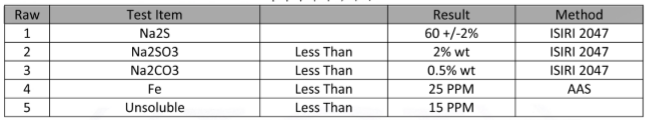
Physical and Chemical Properties of Sodium Sulfide
Sodium sulfide appears in white to pale yellow colors and is produced in various physical forms, including powder, liquid, and crystalline states.
This compound has a strong odor similar to rotten eggs, which is due to the release of hydrogen sulfide gas (H₂S) upon contact with water and air.
Sodium sulfide is highly soluble in water and forms a strongly alkaline solution.
Among its chemical properties, high reactivity is one of the most notable. Sodium sulfide reacts vigorously with acids, producing hydrogen sulfide gas, which is not only highly toxic but also flammable.
Sodium Sulfide Production Line
The production line for sodium sulfide involves the reaction of sodium carbonate (Na₂CO₃) with sulfur in high-temperature furnaces. This process yields sodium sulfide (Na₂S) and carbon dioxide (CO₂) as byproducts.
After cooling, the product is collected in crystalline or powdered form, and purification steps are carried out to increase its quality and purity.
Finally, the sodium sulfide is packaged and made ready for use in various industries such as leather processing, water treatment, paper manufacturing, and chemical production.
Purchasing Sodium Sulfide from Iran
Tehran Sulfur Chemical Industries is one of the largest manufacturers of sodium sulfide in Iran.
For purchase inquiries or to place an order, please contact us at:
+98 21 2204 0205
Info@tehransulphur.com
| Articles |
|
|
| Shortcut |
|
factory add: janat abad industrial park-khavaran road-Tehran-Iran
central office: Tehran, Nelson Mandela Boulevard (Jordan), Niloufar Alley, No. 10, Unit 7, (Kimia Javid Tehran Company)
tel: +98 21 22040205
email: info@tehransulphur.com
Postal code: 1967758458
|
Kimia Javid Tehran.Co . All rights reserved